This vaccine is licensed by the US FDA and has been authorised by the MHRA for temporary supply in the UK to meet public health need for use in the national Immunisation programme. Completed in 2018 Seqiruss Holly Spring NC plant was funded with 59 million from the US.
 Seqirus 66521011810 Mckesson Medical Surgical
Seqirus 66521011810 Mckesson Medical Surgical
Weve been around for.

Seqirus flu vaccine. An influenza A H1N1 virus an influenza A H3N2 virus and two influenza B viruses. Active immunisation against influenza in the elderly 65 years of age and over especially for those with an increased risk of associated complications. Seqirus a global leader in influenza prevention today presented new real-world evidence RWE at the European Scientific Working Group on Influenza ESWI 2020 showing the clinical benefits of a.
Seqirus a global leader in influenza prevention today presented new real-world evidence RWE on the effectiveness of an MF59-adjuvanted trivalent seasonal influenza vaccine aTIV in adults 65. The facility will be a significant addition to the companys global manufacturing and supply chain for the influenza vaccine including the facilities in Holly Springs North Carolina US Liverpool UK and Parkville Australia. DocWire News recently spoke with Dr.
The companys flu business Seqirus delivered revenue growth of around 38 on the back of a 44 increase in flu vaccinations says SP Global. Seqirus Releases New Data in Support of Pandemic Flu Vaccine Although most of us currently are consumed by the COVID-19 coronavirus its also the season to be thinking about the flu. Seqirus USA Inc 70461012003 FLUAD 2020 - 2021 Flu Vaccine Adjuvanted 45 mcg 05 mL Indicated For People 65 Years of Age and Above Prefilled Syringe 05 mL FLUAD 2020 QUAD SYR 05ML 65YEAR ADJ 3PL 10DOSEBX.
The continued presence of COVID-19 has significantly boosted demand for seasonal influenza vaccines. So Seqirus is a fairly new company. In November 2015 BioCSL rebranded the combined business with Novartis Influenza Vaccines as Seqirus Sek-eer-us creating the worlds second largest influenza vaccine company.
The use of Adjuvanted Trivalent Influenza Vaccine Surface Antigen Inactivated should be based on official recommendations. As one of the largest influenza vaccine providers in the world Seqirus is a major contributor to the prevention of influenza globally and a. CSL merged it into its BioCSL operation.
FLUCELVAX QUADRIVALENT is a vaccine that helps protect people aged 4 and older from the flu. Fluad and aTIV-2 containing an alternate B strain examine whether aQIV had immunological superiority for the B strain absent from aTIV comparators and evaluate reactogenicity and safety among adults. Evaluate whether adjuvanted quadrivalent influenza vaccine aQIV elicits a noninferior immune response compared with a licensed adjuvanted trivalent influenza vaccine aTIV-1.
Flucelvax Quadrivalent 2020 - 2021 Flu Vaccine 60 mcg 05 mL Indicated For People 4 Years of Age and Above Prefilled Syringe 05 mL Seqirus USA Inc 70461032003. Gregg Sylvester Chief Medical Officer of Seqirus one of the worlds largest manufacturers of influenza vaccines. Seqirus one of the largest influenza vaccine companies in the world has just announced the release of new data that demonstrate the effectiveness and safety of its pandemic influenza A H5N1 vaccine.
As one of the largest influenza vaccine. Can you talk to us a little bit about Sequirus the the vaccines it produces. Adjuvanted Trivalent Influenza Vaccine Surface Antigen Inactivated Suspension for Injection in Pre-filled Syringe Influenza Vaccine Adjuvanted with MF59C1 - Patient Information Leaflet PIL by Seqirus UK Limited.
Seqirus USA Inc 70461032003 Flucelvax Quadrivalent 2020 - 2021 Flu Vaccine 60 mcg 05 mL Indicated For People 4 Years of Age and Above Prefilled Syringe 05 mL FLUCELVAX QUADRIVALENT is an inactivated vaccine indicated for active FLUCELVAX is approved for use in persons 4 years of age and older. Vaccination with FLUCELVAX QUADRIVALENT may not protect all people who receive the vaccine. About Seqirus Seqirus is part of CSL Limited ASX.
Seqirus an influenza vaccine maker is building a new cell-based influenza vaccine manufacturing facility in Melbourne Australia. Most flu vaccines in the United States protect against four different flu viruses quadrivalent. FLUAD QUADRIVALENT is an inactivated influenza vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and types B contained in the vaccine.
The 140m expansion will enable Seqirus to meet the growing requirements of its cell-based quadrivalent influenza vaccine QIVc. The seasonal flu vaccine protects against the influenza viruses that research indicates will be most common during the upcoming season. Seqirus was established on 31 July 2015 following CSLs acquisition of the Novartis influenza vaccines business and its subsequent integration with bioCSL.
Seqirus a biotherapeutics company based in Australia announced plans to expand its flu vaccine manufacturing facility located in Holly Springs North Carolina US in November 2018.
 Fda Approves Seqirus Fluad Quadrivalent For Adults Over 65
Fda Approves Seqirus Fluad Quadrivalent For Adults Over 65
 Seqirus Begins 140 Million Expansion Of Flu Vaccine Plant North Carolina Biotechnology Center
Seqirus Begins 140 Million Expansion Of Flu Vaccine Plant North Carolina Biotechnology Center
 Seqirus Begins On Time Delivery Of Flu Vaccines Across The Uk Pharma Business International
Seqirus Begins On Time Delivery Of Flu Vaccines Across The Uk Pharma Business International
 Seqirus Usa Inc 70461042010 Mckesson Medical Surgical
Seqirus Usa Inc 70461042010 Mckesson Medical Surgical
 Seqirus Announces Further Advances In Cell Based Influenza Vaccine Technology
Seqirus Announces Further Advances In Cell Based Influenza Vaccine Technology
 Seqirus 62577061401 Mckesson Medical Surgical
Seqirus 62577061401 Mckesson Medical Surgical
 Rx Item Fluad Seqirus Tiv In Pfs 10 Pk 5ml Syringe 2020 21 By Sequiris 65
Rx Item Fluad Seqirus Tiv In Pfs 10 Pk 5ml Syringe 2020 21 By Sequiris 65
Flucelvax Quadrivalent 2019 2020 Flu Vaccine 60 Mcg 0 5 Ml In Allmedtech Com
 Seqirus Usa Inc 70461002003 Mckesson Medical Surgical
Seqirus Usa Inc 70461002003 Mckesson Medical Surgical
 Fluvirin Influenza Vaccine By Seqirus Vessel Medical
Fluvirin Influenza Vaccine By Seqirus Vessel Medical
 Seqirus Influenza Vaccine Manufacturing Facility Melbourne Australia
Seqirus Influenza Vaccine Manufacturing Facility Melbourne Australia
 Seqirus Receives Fda Approval For New Mfg Process Contract Pharma
Seqirus Receives Fda Approval For New Mfg Process Contract Pharma
 Seqirus Starts On Time Shipping Of Influenza Vaccines Across Uk Epm Magazine
Seqirus Starts On Time Shipping Of Influenza Vaccines Across Uk Epm Magazine
 Seqirus Adjuvanted Quadrivalent Flu Vaccine Shows Better Protection For Young Children Fiercepharma
Seqirus Adjuvanted Quadrivalent Flu Vaccine Shows Better Protection For Young Children Fiercepharma
 Seqirus Facility Delivers Four Fold Increase In Seasonal Influenza Vaccine Output In Two Years Homeland Preparedness News
Seqirus Facility Delivers Four Fold Increase In Seasonal Influenza Vaccine Output In Two Years Homeland Preparedness News
Afluria Quadrivalent 2017 2018 Flu Vaccine 60 Mcg 0 5 Ml Indi Allmedtech Com
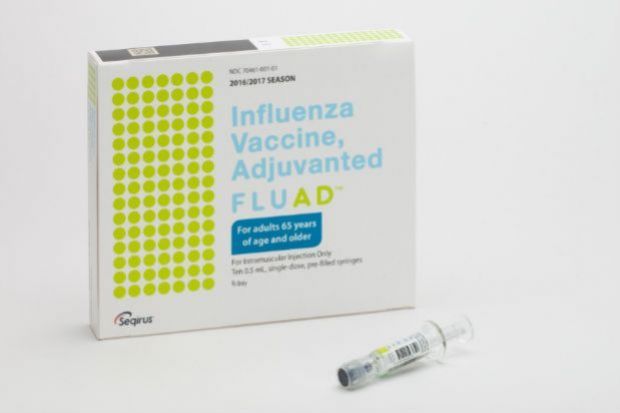 Manufacturer Processing Challenges Affected Some Flu Vaccine Orders Chemist Druggist
Manufacturer Processing Challenges Affected Some Flu Vaccine Orders Chemist Druggist
 Glaxosmithkline Seqirus Launch First Round Of Flu Shots For Upcoming Season Fiercepharma
Glaxosmithkline Seqirus Launch First Round Of Flu Shots For Upcoming Season Fiercepharma
Search This Blog
Blog Archive
- February 2021 (11)
- December 2019 (4)
- November 2019 (4)
- October 2019 (8)
- September 2019 (14)
- August 2019 (1)
- July 2019 (26)
- June 2019 (161)
- May 2019 (25)
- November 2018 (10)
- October 2018 (33)
- September 2018 (46)
- August 2018 (82)
- July 2018 (255)
- June 2018 (26)
- May 2018 (14)
- April 2018 (11)
- March 2018 (83)
- February 2018 (97)
- November 2017 (44)
- October 2017 (13)
- September 2017 (78)
- June 2017 (32)
- May 2017 (27)
- March 2017 (26)
- February 2017 (122)
- January 2017 (360)
- December 2016 (1027)
- November 2016 (285)
Labels
- 1000
- 1000rr
- 1080p
- 11x14
- 12th
- 12x36
- 1366x768
- 13th
- 1500
- 15000
- 1600x900
- 1701
- 18th
- 18x18
- 1920x1080
- 1994
- 1999
- 2000
- 200cc
- 2015
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 30th
- 3840x2160
- 4000k
- 40th
- 45th
- 50th
- 8962
- abon
- abstract
- academia
- acar
- acceptance
- accord
- account
- accounting
- acdc
- acoustic
- acquisition
- across
- acrylic
- Action
- activities
- actress
- addition
- address
- adhesive
- adidas
- adjustments
- adrenalin
- adults
- advantages
- adventures
- aerial
- aesthetic
- affected
- affiliate
- affordable
- africa
- after
- ages
- agreement
- aircraft
- airplane
- alarm
- album
- alchemy
- alcohol
- allianz
- allotment
- allow
- almond
- alola
- alone
- alpha
- alphabet
- aluminum
- amanda
- amazing
- amazon
- ambe
- american
- amman
- amortization
- amsterdam
- anak
- analyse
- android
- aneka
- angeles
- animal
- animals
- animated
- anime
- anjaneya
- anniversary
- answers
- anti
- antibody
- apertura
- apple
- apples
- application
- apply
- approve
- april
- aquarium
- aquatic
- arabic
- arches
- architectural
- areas
- arena
- arhaus
- armitage
- articles
- articolo
- artistic
- artsy
- artwork
- asam
- asem2
- asian
- asin
- asinan
- astrazeneca
- astronaut
- atlanta
- atlas
- atop
- attenuated
- audi
- audible
- australia
- auto
- autoimmune
- autumn
- avatar
- avengers
- away
- ayam
- azerbaijan
- baba
- babies
- baby
- bacem
- back
- backdrops
- background
- backgrounds
- backlit
- backyard
- bagon
- bahamas
- bajaj
- bakar
- bake
- bakpia
- bakso
- bakwan
- balado
- balaji
- bali
- ball
- ballet
- balloon
- bamboo
- banana
- band
- bands
- baroque
- barrel
- basah
- baseball
- basic
- basketball
- baso
- bathroom
- batman
- battery
- bawang
- bayi
- beabadoobee
- beach
- bean
- bear
- beat
- beatles
- beautiful
- beauty
- bechamel
- become
- bedroom
- bedrooms
- beef
- been
- beginner
- beginners
- beginning
- bein
- bekas
- bench
- benefits
- beng
- bening
- beras
- berlin
- best
- betawi
- betty
- between
- bhagwan
- bhai
- bible
- bicycle
- bigger
- bike
- bikin
- bill
- biohazard
- bioshock
- biotech
- birch
- bird
- birds
- birthday
- birthdays
- bistik
- black
- blade
- blanche
- blank
- blastoise
- blend
- blessings
- blizzard
- block
- blossom
- blue
- blueprint
- blush
- board
- boba
- body
- bola
- bolas
- bollywood
- bolognese
- bolu
- book
- books
- boost
- booster
- boots
- border
- borders
- boss
- both
- bottle
- bouncy
- bouquets
- boxes
- boys
- brainly
- brakes
- brand
- branded
- brazzers
- bread
- break
- breakdancing
- breaking
- breathing
- brick
- bride
- brides
- british
- bronco
- broncos
- brongkos
- bronze
- brooklyn
- bros
- brothers
- brownies
- browning
- brownis
- brushes
- buah
- buat
- buaya
- bubur
- buddha
- budget
- buka
- bull
- bumbu
- buncis
- bunny
- buntil
- buntut
- burden
- burger
- burgers
- burning
- busch
- business
- butterfly
- buzzfeed
- cabe
- cable
- cafe
- cagr
- cake
- calculator
- calendar
- california
- call
- calligraphy
- calories
- calves
- calvin
- camaro
- camera
- cameras
- campur
- cancel
- cancelled
- canine
- canvas
- canyon
- capcay
- cara
- carbonara
- card
- cards
- care
- cargo
- cari
- carnival
- carpet
- carrera
- cartoon
- carved
- case
- cases
- casio
- cast
- catalytic
- catch
- cats
- cave
- cavities
- ceker
- celerie
- cell
- cellular
- cement
- cemilan
- cendol
- centerpiece
- cepat
- ceramic
- ceremony
- certificate
- chacos
- chair
- chairs
- chakra
- chameleon
- championship
- change
- changer
- charger
- chargers
- charizard
- chart
- cheap
- checklist
- cheese
- cheetah
- cherry
- chess
- chick
- chicken
- chickenpox
- child
- childrens
- chip
- chloroplasts
- chocolate
- chopper
- chords
- chris
- christian
- christmas
- chromebook
- chubb
- church
- churros
- cigar
- cincang
- cincau
- cinnamon
- circle
- circular
- cireng
- cite
- cities
- citizen
- city
- civic
- classic
- clean
- cleaning
- clinical
- clip
- clips
- clock
- close
- cloth
- clothes
- cloud
- club
- coastal
- coats
- cockpit
- cockroaches
- cocktail
- code
- codes
- coffee
- coffin
- coklat
- cold
- collage
- collection
- college
- color
- colorful
- coloring
- colors
- column
- come
- commercial
- common
- community
- company
- compare
- compass
- compliment
- comprehension
- computer
- concrete
- conditioner
- conditioning
- cone
- confetti
- confused
- connects
- consent
- consolidation
- constellation
- contact
- contacto
- contagious
- container
- conte
- contemporary
- contract
- contractor
- controller
- convertible
- cool
- coordinates
- copyright
- core
- coronavirus
- corporate
- corriere
- corvette
- cost
- costume
- costumes
- cottage
- cottagecore
- couch
- cough
- countdown
- countertops
- country
- couple
- couples
- coupon
- coupons
- cousin
- cover
- covers
- covid
- cracked
- crafts
- crayola
- crayon
- creamer
- create
- created
- creator
- crest
- cricket
- cricut
- crispy
- crocodile
- croissant
- cross
- crossing
- croup
- cuanki
- cube
- cumi
- cumi2
- cursive
- custom
- customize
- cute
- cutting
- da2pp
- daerah
- daging
- dalgona
- dali
- damask
- dance
- dara
- dark
- dart
- dashboard
- date
- daun
- davidson
- daycare
- dead
- deadlift
- deadpool
- deal
- deals
- debm
- decals
- decathlon
- december
- decimals
- decline
- deco
- decoding
- decor
- decorating
- decorations
- definition
- degreee
- dehydrate
- delivery
- demon
- denali
- dendeng
- dennys
- dental
- dentist
- denver
- depopulation
- desert
- design
- designer
- designs
- desk
- desks
- desktop
- destiny
- details
- detective
- detox
- deutsch
- developer
- development
- devi
- deviated
- device
- diapers
- dibuat
- diet
- different
- digit
- digital
- dimensional
- dimensions
- dining
- dinner
- dinosaur
- direct
- direction
- directions
- discount
- disease
- disinfektan
- disney
- display
- distemper
- distribution
- disturb
- dividing
- divorce
- doboj
- doctor
- dodd
- dodol
- does
- dogs
- dokter
- dolly
- dolphin
- donuts
- door
- dorayaki
- dose
- dota
- dotted
- double
- down
- download
- dragonite
- drain
- dratini
- draw
- drawing
- drawings
- dress
- dresses
- drill
- drills
- drive
- drivers
- drywall
- dual
- dualshock
- dubai
- duck
- ducks
- dulhan
- dungeness
- dunkin
- durga
- durian
- during
- duty
- dylan
- dynamic
- dysgraphia
- easter
- easy
- edge
- edging
- edgy
- edition
- editor
- edmunds
- eevee
- effect
- effective
- effects
- eggs
- egypt
- eight
- electric
- elegant
- elephant
- elite
- embroidery
- emerald
- emergency
- emoji
- employment
- empuk
- enable
- enak
- endangered
- engine
- english
- enterprise
- enterprises
- epic
- equation
- equations
- equestrian
- escher
- etsy
- european
- evening
- ever
- everything
- evolutions
- evoque
- exam
- examples
- excel
- exclusives
- exercise
- exercises
- exotic
- expensive
- explorer
- extenze
- extinct
- extreme
- eyeglass
- eyewash
- fabric
- face
- facetime
- fairy
- faith
- fajar
- fake
- fall
- Family
- farley
- farm
- farmhouse
- fast
- fates
- feather
- feeder
- feelings
- feet
- feline
- fence
- fencing
- ferm
- fettuccine
- fever
- field
- file
- fillable
- filter
- financial
- find
- finding
- finetec
- fire
- firestick
- first
- fish
- fist
- flag
- flamingo
- flip
- flock
- floor
- floral
- florida
- floss
- flower
- flowers
- fluffy
- flying
- foam
- following
- footage
- football
- footnotes
- ford
- foreign
- forest
- forester
- form
- forms
- formula
- formulas
- fortnite
- fossils
- foto
- fountain
- four
- foyer
- fractions
- framed
- frames
- france
- free
- freeze
- french
- friend
- friendly
- friends
- frog
- from
- front
- frontgate
- fruit
- full
- fundamental
- funny
- fuyunghai
- fvrcp
- gaara
- gabus
- gagal
- galaxy
- gallery
- galt
- gambar
- game
- games
- gamestop
- gaming
- ganesh
- garage
- garden
- gardens
- garing
- gates
- gauntlet
- gautam
- gautama
- gears
- gehu
- geico
- general
- generations
- generator
- geometric
- geometry
- george
- geplak
- giants
- gift
- gifts
- gimbal
- gingerbread
- girl
- girls
- give
- given
- giving
- glass
- glossy
- glyphosate
- gnome
- goddess
- gogh
- gold
- goldfish
- golf
- gonore
- good
- goodnight
- goreng
- gospel
- gossip
- gotten
- gown
- gowns
- goyang
- grace
- grade
- graders
- graffiti
- grammar
- gran
- grandpa
- graphs
- grass
- gratis
- gravel
- gray
- great
- green
- grey
- grid
- grinch
- grinder
- ground
- guatemala
- gudeg
- guide
- guitar
- gulab
- gulai
- gummies
- gurame
- guys
- hacker
- hair
- halloween
- hallway
- hand
- handheld
- handicap
- handwriting
- hanging
- hangings
- hanuman
- happily
- happy
- harga
- hari
- harry
- have
- havertys
- head
- header
- headlines
- headphones
- heads
- headshot
- heal
- health
- hearth
- heat
- hedge
- heels
- height
- hendrix
- herb
- herbivores
- hero
- heroine
- heroines
- heron
- hers
- hibiscus
- hidden
- high
- highland
- highschool
- hijau
- hindi
- hippie
- hipster
- hiroin
- History
- hobbes
- hobby
- holder
- holes
- holiday
- holidays
- hollywood
- home
- homemade
- homeopathic
- homeschool
- homework
- honda
- hong
- hook
- horizontal
- horse
- house
- housewarming
- houston
- howard
- hoyo
- hubble
- human
- humana
- humans
- humble
- hummingbird
- hunt
- hunter
- husband
- icing
- idea
- ideas
- ikan
- illuminated
- illustration
- image
- images
- improve
- inch
- inches
- income
- independent
- india
- indian
- indigo
- indonesia
- indoor
- infinity
- influenza
- information
- infused
- ingredients
- injection
- injuries
- injury
- inovio
- inspiration
- inspirational
- installation
- instan
- instructions
- insurance
- intermediate
- into
- invitation
- ipad
- iphone
- ireland
- islami
- islamic
- issues
- istimewa
- italian
- itunes
- jack
- jackson
- jacob
- jagung
- jailbreak
- jajanan
- jakarta
- jamu
- jamur
- janeiro
- jantung
- january
- japan
- japanese
- jeans
- jehovah
- jengkol
- jesus
- jewish
- jimmy
- jobs
- jogja
- joker
- jordan
- jornal
- journal
- journaling
- jualan
- juice
- jumper
- justice
- kacang
- kakkar
- kambing
- kandi
- kangkung
- kansas
- kanye
- kappa
- karat
- karate
- karedok
- kawaii
- kawasaki
- kebuli
- kecap
- keep
- keju
- kekinian
- keldeo
- kembang
- kemble
- kendrick
- kentang
- kepiting
- kerang
- kerapu
- keren
- kering
- keripik
- ketan
- keto
- ketupat
- keurig
- keychain
- keypad
- khas
- kids
- kikil
- kill
- kindergarten
- kindle
- kinkade
- kirby
- kirklands
- kitchen
- kitten
- kittens
- kiwi
- klapetart
- klepon
- kobe
- koki
- kong
- kontakt
- korea
- kreni
- krishna
- krispi
- krokorok
- kuah
- kukus
- kulit
- kuning
- kupas
- kuretake
- kwetiau
- kyogre
- label
- labels
- labrador
- labs
- labu
- ladies
- laid
- lake
- lakshmi
- lama
- lamar
- lamborghini
- lana
- lanai
- land
- landscape
- lantern
- laptop
- large
- lasagna
- last
- latest
- latte
- laughing
- laut
- lava
- lavender
- laxmi
- layer
- layers
- leaf
- leafeon
- league
- learn
- learning
- leave
- leaves
- ledger
- legendary
- lego
- leker
- lele
- lembang
- lembut
- lenovo
- lessons
- letter
- letters
- level
- lexus
- liability
- libra
- library
- license
- lidah
- liem
- life
- light
- lights
- like
- lilies
- lily
- limit
- line
- link
- lion
- lips
- lisa
- list
- litter
- little
- live
- liverpool
- living
- livorno
- liwet
- lobby
- location
- locations
- locator
- lodge
- logic
- login
- logo
- london
- long
- lontong
- look
- looks
- loosen
- lord
- lords
- lotus
- lounge
- love
- lovers
- lower
- lowes
- lumer
- lumpia
- lumpur
- lyrics
- macbook
- macdermot
- machine
- madura
- magic
- magnet
- magnifying
- magyar
- mahalaxmi
- main
- makanan
- makaroni
- make
- maker
- makes
- malayalam
- malaysia
- mandala
- mangga
- manis
- manisa
- many
- maranggi
- marble
- mario
- markers
- marketplace
- marmer
- maroon
- marriage
- marshmello
- martabak
- marthe
- marvel
- mary
- masak
- masakan
- masculine
- mashed
- masks
- mata
- mataji
- maternity
- math
- maths
- matrix
- matter
- mattress
- maxi
- mazda
- meaning
- measles
- measure
- mechanism
- medan
- medical
- mediterranean
- medium
- mega
- mehandi
- melts
- membuat
- membuka
- meme
- memes
- menb
- mendut
- meningitis
- menstruasi
- menu
- mercon
- mercury
- meriah
- mermaid
- mesh
- messages
- messenger
- metal
- metallic
- metropolitan
- mewtwo
- mexican
- miata
- michaels
- michigan
- microsoft
- millers
- milo
- mind
- mini
- minimalist
- minnie
- mitochondria
- mixer
- mixing
- mobile
- moci
- mockup
- model
- modern
- moderna
- monet
- money
- monopoly
- monster
- montgomery
- month
- monthly
- moon
- mooncake
- moose
- morning
- moss
- most
- motivational
- motor
- motorcycle
- mountain
- mouse
- moveset
- movie
- moving
- mpasi
- mrcoffee
- mrna
- much
- mudah
- multiplication
- mural
- murals
- muriels
- murugan
- Music
- mustang
- mustard
- nachural
- naga
- nagasari
- nails
- name
- names
- narrative
- nasi
- nastar
- national
- nativity
- nature
- nautical
- navy
- near
- need
- neha
- nencini
- neon
- netflix
- network
- newborn
- newest
- News
- next
- niantic
- nick
- nicol
- night
- nightmare
- ninja
- nintendo
- nissan
- normal
- northern
- note
- nova
- novavax
- nugget
- number
- numbers
- nyquil
- obat
- obvio
- ocean
- octopus
- office
- officiant
- offline
- often
- oggi
- olahan
- olds
- oled
- olshop
- ombre
- oncom
- online
- only
- ontario
- opinion
- oral
- orange
- orari
- order
- origami
- origin
- original
- ornamental
- otak
- ought
- outdoor
- outlet
- outline
- outlook
- oven
- oversized
- owners
- pack
- packs
- padang
- page
- pages
- pagoda
- paint
- paintable
- painting
- paintings
- pakistani
- palembang
- palm
- panasonic
- pancake
- panchamukhi
- panda
- pandan
- panels
- panggang
- paper
- paragraphs
- pare
- parents
- park
- parking
- part
- party
- paru
- parvati
- paste
- pastel
- patch
- patent
- patio
- patriotic
- pattern
- peanuts
- peel
- pelangi
- pentol
- penyet
- peony
- pepes
- permen
- pernikahan
- perth
- pexels
- pfizer
- philadelphia
- philip
- philosophy
- phone
- phones
- photo
- photographers
- photography
- photos
- physics
- piana
- piano
- pick
- pics
- picture
- pictures
- piece
- pigs
- pikachu
- pindang
- pine
- pink
- pisang
- piscok
- pitbull
- pixabay
- pixel
- pixellab
- place
- plague
- plan
- plane
- plank
- planner
- plant
- plants
- plates
- play
- playboy
- playing
- plus
- pneumococcal
- pneumonia
- pods
- poem
- pokemon
- polio
- polish
- politely
- pond
- pong
- ponies
- pool
- poppers
- porcelain
- porsche
- portable
- portrait
- poster
- potato
- potatoes
- pothos
- pounds
- pouring
- powers
- practice
- prague
- pray
- precision
- predictions
- pregnancy
- premier
- preparedness
- preschool
- preserve
- press
- prettiest
- pretty
- prevent
- prevnar
- price
- prices
- prima
- primer
- primitive
- prince
- printable
- printables
- printer
- prints
- probiotics
- problem
- problems
- procedural
- process
- product
- production
- professional
- progressive
- project
- promil
- promissory
- promo
- pronounce
- proof
- propeller
- properly
- protection
- protocol
- prussian
- psycho
- psychology
- puasa
- publish
- pudding
- puding
- pulsar
- pumps
- punctuation
- puppy
- purple
- putih
- putu
- puzzle
- puzzles
- pyramids
- quadrilateral
- quality
- queen
- quest
- quick
- quiz
- quora
- quote
- quotes
- rabbit
- rabies
- racing
- radha
- radios
- rainbow
- rainforest
- raised
- range
- rangoli
- rapper
- rate
- razor
- read
- reading
- real
- realistic
- reality
- reception
- recipe
- recipes
- recommendations
- record
- recover
- reduce
- regional
- registry
- regrouping
- reindeer
- related
- release
- remix
- removable
- remove
- remover
- rendang
- rendering
- rent
- rental
- renters
- renyah
- reporting
- repot
- repsol
- resep
- resolution
- respiration
- retailers
- retrieve
- retro
- review
- reviews
- rhinestone
- rica
- rice
- rich
- rings
- ripe
- roadhouse
- roast
- robert
- rock
- rocks
- rocky
- rogers
- rogue
- roll
- roller
- romantic
- roof
- room
- rooms
- rose
- roseville
- ross
- roti
- rover
- royalty
- rujak
- rules
- rustic
- sacred
- sage
- sagging
- salad
- salatiga
- saldi
- sale
- salju
- salted
- sambal
- sambalado
- samcan
- samoan
- samsung
- sanei
- sanitizer
- sans
- santan
- santri
- saori
- sapi
- saree
- sassy
- saus
- sausage
- sauth
- sawi
- sayur
- scandi
- scarface
- scavenger
- scenery
- schedule
- scheme
- schools
- schotel
- science
- scottish
- scrabble
- screen
- screensaver
- screensavers
- script
- scroll
- sculpture
- seafood
- search
- season
- seattle
- seblak
- second
- secret
- secure
- security
- sederhana
- sekolah
- selar
- semarang
- semprit
- semur
- seniors
- sentences
- seqirus
- sequencing
- serangan
- series
- sets
- seven
- seventh
- shade
- shadi
- shadows
- shampoo
- shankar
- shapes
- share
- shark
- sheets
- shield
- shingle
- shingles
- shiny
- ship
- shirt
- shirts
- shiv
- shiva
- shoes
- shop
- shopify
- shops
- Short
- show
- shree
- shri
- shutter
- side
- sight
- sign
- signature
- signs
- sikaporo
- sikkim
- silver
- simpel
- simple
- simulator
- singapore
- singer
- singkong
- single
- singset
- siomay
- sister
- site
- sites
- sitting
- sitton
- sixth
- size
- sizer
- skateboard
- skills
- skinny
- skull
- skyline
- slayer
- sleep
- slice
- slim
- small
- smart
- smartwatch
- smile
- smoking
- snake
- snapchat
- snowflake
- snowmobile
- social
- society
- society6
- sofia
- soldier
- solid
- solo
- song
- songs
- sony
- sosis
- soto
- sound
- sources
- south
- space
- spacex
- spalding
- spanish
- spawn
- spec
- specs
- spectate
- spectra
- spell
- spelling
- spice
- splash
- sponge
- spongebob
- Sport
- sports
- sprinkler
- sprint
- square
- squirtle
- stacked
- stadium
- stag
- stains
- stand
- standing
- stardust
- starry
- starship
- start
- starters
- states
- station
- stationery
- statue
- steam
- steampunk
- steel
- steelers
- stencils
- step
- steps
- stern
- stick
- stickers
- stiletto
- stilettos
- still
- stock
- stone
- storage
- store
- story
- strait
- stranger
- stream
- streaming
- street
- stretch
- strip
- stripe
- struggles
- students
- studies
- studio
- study
- stup
- style
- styles
- stylish
- suami
- subaru
- subscript
- substitution
- subunit
- suburban
- succulent
- succulents
- suffix
- sugar
- summer
- sunburst
- sunda
- sunflowers
- sunrise
- sunset
- super
- superb
- superman
- superstore
- supply
- supreme
- surf
- surgery
- susu
- swamy
- swastik
- sweatshirt
- sweden
- switch
- switches
- sword
- symbol
- symptoms
- system
- tabby
- table
- tablet
- tabs
- taehyung
- tags
- tahu
- tahun
- take
- takes
- taking
- talam
- talking
- tamil
- tanak
- tank
- tanpa
- tape
- target
- tarot
- tassimo
- tattoo
- tattoos
- tdap
- teach
- teacher
- teaching
- team
- tech
- techniques
- technology
- teen
- teenage
- teenager
- teflon
- teler
- television
- tell
- telugu
- telur
- tempe
- template
- templates
- temporary
- tennis
- tepung
- terasi
- terbaru
- teri
- teriyaki
- terong
- terrazzo
- terus
- test
- testament
- tested
- tetanus
- texas
- text
- thai
- thailand
- thanksgiving
- that
- theater
- there
- thermos
- things
- third
- thomas
- threatened
- three
- through
- throughout
- tidak
- tier
- tiktok
- tile
- tiles
- time
- timed
- times
- timlo
- tint
- tiny
- tire
- toile
- toilet
- tomyam
- tone
- tongseng
- tools
- topper
- topsy
- tortilla
- totoro
- tour
- touring
- toyota
- tracing
- tracker
- tracking
- tractor
- traditional
- trailer
- trainer
- transfer
- transfers
- transgenic
- transparent
- trap
- travel
- treat
- treatment
- tree
- trends
- tressa
- trials
- tricks
- tropical
- trump
- trunk
- tuberculosis
- tuesday
- tulang
- tumis
- tune
- turismo
- turkey
- turtles
- turvy
- tutorial
- tutug
- twig
- twitch
- typhoid
- udang
- uefa
- ugliest
- ugly
- ukulele
- ultra
- unblock
- unblur
- under
- underwear
- unicorn
- unicorns
- union
- unit
- unlimited
- unlocked
- unsplash
- unsubscribe
- untuk
- update
- urap
- urban
- vaccinated
- vaccination
- vaccinations
- vaccine
- vaccines
- value
- vanilla
- vape
- vaporwave
- varicella
- vector
- vegetable
- veggie
- vehicle
- veil
- venice
- venkateswara
- venue
- veterans
- victorian
- video
- videos
- vimeo
- vincent
- vintage
- vinyl
- vlog
- vocabulary
- voice
- volcano
- vowel
- wall
- wallet
- wallpaper
- wallpapers
- walls
- walmart
- want
- ward
- warm
- warranty
- wars
- washable
- watch
- watches
- water
- watercolor
- watercolors
- wayfair
- weather
- weave
- website
- webstore
- wedding
- weed
- week
- weight
- wexford
- what
- when
- where
- which
- whimsical
- white
- whitewash
- whooping
- wide
- widescreen
- wife
- wikipedia
- wild
- wildlife
- will
- window
- windows
- wine
- wings
- winter
- wireless
- wise
- wishes
- with
- without
- witness
- woku
- wolf
- woman
- women
- wonder
- wonderful
- wood
- wooden
- word
- words
- work
- worksheet
- worksheet_change
- worksheets
- workshop
- world
- worlds
- wortel
- woven
- wrap
- wrinkle
- write
- writing
- wrld
- xanax
- yang
- yantra
- yard
- year
- yeast
- yeezy
- yellow
- yoni
- york
- your
- youtube
- yupo
- zebra
- zinsser
- zombies
- zone
- zoom
-
20 Off Dennys Coupons Printable Coupons February 2021. Dennys Coupons Deals Printable CouponShy 20 Restaurant Discounts for Grand Slam Dinin...
-
The main aim behind this documentation is to provide complete information for research and analysis of different species. More birds are at ...
-
The vaccine appeared to be more or less equally protective across age groups and racial and. After releasing positive interim. Covaxin And...

